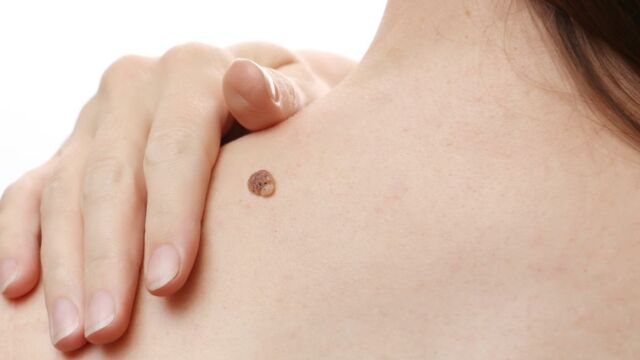
The Food and Drugs Administration in the US has ordered Amazon and two other retailers to desist from selling unapproved brands of mole and skin treatment products, DailyMail reports. The FDA said the products have not been tested for safety and effectiveness and should not be sold to the public.
Discover our latest podcast
Unnamed products
The warning, issued last week was to the retail giant as well as Ariella Naturals and Justified Laboratories. In a statement announcing the warning, the FDA said skin lesions and moles cannot be removed or treated by over-counter products.
Removing them (skin lesions and moles) isn’t a do-it-yourself project, and it can be dangerous to try. Please see a health care provider to have them evaluated and removed, if necessary.
What this means is that, it is illegal to sell products —including creams, gels and ointments— purporting to treat these skin conditions without prescription.

Risks
In fact, the FDA has cautioned that using such unapproved and untested products could result in moderate to severe side effects such as skin injuries, infections, scarring and delayed skin cancer diagnosis and treatment, the statement said.
In fact, the FDA has received reports about people who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers.
The regulator added that some of the skin conditions people buy these products to treat are hardly harmful.
According to DailyMail, Amazon said it has removed the affected products from its warehouses and online shops in accordance with the FDA’s warning.
Safety is a top priority at Amazon. We require all products offered in our store to comply with applicable laws and regulations. The products in question have been investigated and removed.
Read more:
⋙ Amazon UK to increase prices for Prime services by 12.5%
⋙ Amazon to stop weeding out job applicants who smoke marijuana
⋙ The type of mole on your body could be an early sign of skin cancer