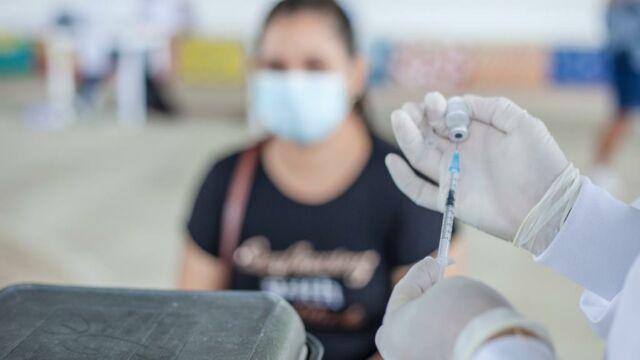
Since the AstraZeneca vaccine was first concocted, coronavirus has mutated several times and now there are a number of different strains on the loose. To combat existing variants, and those to come, researchers at the University of Oxford have been modifying the AstraZeneca vaccine to fight specific variants of concern—the first being the Beta variant that was first identified in South Africa.
Discover our latest podcast
Beta variant vaccine
Yesterday, they inoculated the first bunch of participants with the adapted Beta vaccine called AZD2816. The jab is being tested on 2,250 volunteers from the UK, Poland, Brazil, and South Africa who have already gotten both their COVID doses at least three months prior to trial.
What’s interesting is that while testing the effectiveness of the Beta variant vaccine, the trial will also simultaneously assess the possibility ofmixing different vaccines, given that their participants have either gotten the original AstraZeneca dose or one of the mRNA vaccines. If the results are successful, the jab will be included in the upcoming booster programme.
So far, the vaccine has only been tested on mice and researchers concluded that:
AZD2816 is immunogenic after a single dose and when used as a booster dose in animals primed with [the] original vaccine.
Staying ahead of emerging variants
Sir Mene Pangalos, the Executive Vice President of BioPharmaceuticals R&D at AstraZeneca has said that staying ahead of the variants is imperative and that these new vaccines should strengthen immunity against all types of mutations and strains. He said:
AZD2816 should help broaden individuals' immune response against emerging variants of concern.
The bio-pharmaceutical company has said that although they’ve made subtle changes to the vaccine, the two are more or less the same. They added a few adjustments to the spike protein to replicate what they have seen in the other variants. They explained:
The Beta variant vaccine contains 10 changes across the spike protein, many of which are also seen in other variants of concern, and which lead to effects such as, reduced ability of antibodies induced against the original virus to block cell entry, increased infectivity compared to the original virus, reduced sensitivity of neutralising antibodies to the original virus.